Paul G Mullins PhD
This is an early draft of an article I am submitting for review. It grew out of a presentation on functional magnetic resonance spectroscopy where I discuss the implications and design considerations the MEGA-PRESS acquisition causes for fMRS experiments. This work has also recently been presented at the fMRS symposium in Bangor.
Introduction
There has been an increased interest in the application of functional magnetic resonance spectroscopy (fMRS) to study brain metabolism. fMRS works through the sequential acquisition of magnetic resonance spectroscopy (MRS) during a “task” driving brain activation. The fMRS data is either collected as sequential blocks of a set number of MRS shots (or transients), or in the case of event related fMRS, as single shots (or transients) and then “binning” in some fashion across the time series. Usually spectral transients are binned by “blocks” that coincide with blocked tasks. Alterations in the neurotransmitters glutamate and gama-amino-butyric acid (GABA) have been observed using these sorts of binning techniques with blocked paradigms ((Bednařík et al., 2015, 2017; Chen et al., 2017; Cleve et al., 2014; Ip et al., 2017; Mangia et al., 2008; Mullins, 2018; Mullins et al., 2005; Pasanta et al., 2022; Stanley et al., 2017; Taylor et al., 2015).
Recently, event related dynamics of glutamate have also been investigated using fMRS, and it has been possible to observe, rapid short lived increases in glutamate as fast as 200 ms after stimulus onset (Apšvalka et al., 2015; Lally et al., 2014; Mullins, 2018; Rogan et al., 2022). While this is still an active field, with researchers still interested in glutamate dynamics, more and more researchers are also wanting to investigate inhibitory processes through GABA dynamics using similar event related techniques (Cleve et al., 2014; Koolschijn et al., 2021; Kurcyus et al., 2018).
MRS measures of GABA are usually performed either at high field (7T), where improved spectral resolution allows single transient techniques like PRESS, STEAM, or sLASER to be applied, or at the lower field strength of 3T, where edited sequences like MEGA-PRESS (Mescher et al., 1998; Mullins et al., 2014) dominate. MEGA-PRESS is a dual shot technique, whereby one transient/spectrum is acquired with a frequency selective editing pulse applied to the GABA moieties at 1.9 ppm (the EDIT ON transient), and the other transient/spectrum is acquired with the frequency selective pulse applied further up-field, in a region of the spectral range without any coupled moieties (the EDIT OFF transient). The 1.9 ppm selective pulse of the EDIT ON transient has the effect of refocusing j-evolution of the GABA peaks at 3 ppm, such that all the peaks of this triplet are pointing up, in contrast to the OFF transient, when there is no refocusing of j-evolution and the two outer peaks of the triplet are now 180° out of phase with the central peak (at the typical echo time (TE) used in a MEGA-PRESS acquisition of 68 – 80 ms). (see figure 1). Subtraction of the OFF from the ON spectrum, then leads to the central peaks cancelling out, and the two side peaks, producing a pseudo doublet. When applied in vivo peaks from other metabolites that are not affected by the selective excitation pulse are “edited” out by this subtraction, leaving a much-simplified spectrum (Mescher et al., 1998; Mullins et al., 2014; Puts & Edden, 2012) with the GABA peak now visible and able to be fit reliably ((Evans et al., 2010; O’Gorman, Edden, et al., 2011; O’Gorman, Michels, et al., 2011). This dual shot nature of the MEGA-PRESS sequence therefore means some thought is required before it can be readily applied to event related fMRS paradigms.

Of particular interest is what effect a neurological event that causes a short-lived increase in GABA will have on the individual MRS transients, that is, if that increase happened either during the ON acquisition, the OFF acquisition, or lasted across both. Using simulated spectral acquisitions for both the ON and OFF transients, one can easily test what the effect of a 10% or 20% increase in GABA during either (or both) transients would be on the resulting “edited” subtraction spectrum, by performing a number of different subtractions.
Methods
Simulated spectra were created using the fidA toolbox (Simpson et al., 2015) in Matlab (Matlab, 2022), and the “run_simMegaPressShaped” function, creating a typical MEGA-PRESS spectrum for GABA editing. The sequence in question is based on the Seimens implementation of MEGA-PRESS, and simulates the spectrum at 3T using shaped pulses from a 3 x 3 voxel, accounting for chemical shift effects as an 8 x 8 grid, with EditOn frequency set to 1.9 ppm, and edit off set to 7.5 ppm, with 2048 points across a spectral width of 2000 Hz. Then a vector was produced from the Fourier transform of the real part of the spectrum, which was smoothed by an 18 Hz gaussian kernel to more closely approximate the line shape experienced in vivo. Each sub-spectrum was then multiplied by either 1.1 or 1.2 for the 10% and 20% increases respectively.
Subtraction spectra where then created for [GABA] at baseline, a 10%, and a 20% increase from baseline, for three different scenarios:
- An increase in [GABA] that occurs/persists across both the On and OFF acquisition;
- An increase in [GABA] that only occurs/persists during the ON acquisition;
- An increase in [GABA] that only occurs/persists during the OFF acquisition.
Each resulting edited spectrum was then visually inspected as well as integrated across the 2.5-3.5 ppm range to determine the effect of each proposed scenario on the main edited GABA peak.
Results
The simulated ON and OFF sub-spectra, and the effect of a modelled increases in [GABA] can be seen in figure 2, with a baseline spectrum in black, and a modelled 10% (dashed) and 20% (dot-dashed) increase in [GABA] overlayed on top.

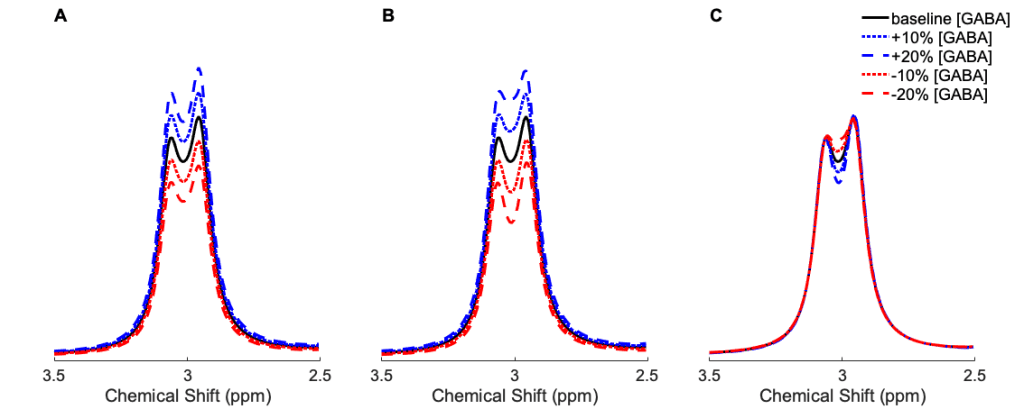
The resulting 3 ppm pseudo-doublet for each of the three proposed scenarios for an increase in [GABA] from an event related stimulus paradigm are shown in figure 3.
From Visual inspection it can be seen that an increase in [GABA] that occurs during the ON acquisition alone, or during both the ON and OFF acquisitions, will give rise to an increase in the GABA peak in the subtraction spectrum, but a [GABA] increase that only occurs during the OFF acquisition leads to a slight decrease in the central part of the subtraction spectrum.
Integration of the area under the curve shows that:
- for the case where [GABA] increases during both the On and OFF acquisitions, the resulting subtraction peak increases by the same amount (10% or 20%);
- for the case where [GABA] is only increased during the ON acquisition, the resulting subtraction peak also increases, but by slightly more than the [GABA] (10.7% and 21.5%)
- for the case where [GABA] increases only during the OFF acquisition, the resulting subtraction peak doesn’t change much, but it does decrease slightly (-0.7% and -1.5%)
Discussion
These modelled results strongly argue that controlling, or at least being aware of, the timing of stimulus onset in relation to transient acquisition time, is crucial in fMRS studies utilizing MEGA-PRESS for data acquisition. While this is especially pertinent when event related designs are being used, and a “fast” GABA response is theorised, the impact of the timing of [GABA] changes on the different portions of the MEGA-PRESS acquisition (and other dual shot techniques) will impact all MEGA-PRESS based fMRS studies of GABA, and should be considered when designing both the paradigm, and the acquisition strategy. For example, even for block related designs, these results suggest that detection of [GABA] increases will be maximised if the on condition for the task and the ON transients of the MEGA-PRESS acquisition are synchronised.
A counter argument to these concerns may be that the MRS visible GABA changes are too slow and persistent for these concerns to really be an issue. However, in the absence of a known “GABAergic response function” detailing the response of GABA’s rise and fall in response to single stimuli, it would still be prudent to consider the impact of stimulus timing on expected results, and design experiments (stimulation and acquisition timing) accordingly.
Some design considerations for fMRS experiments using MEGA-PRESS for GABA:
- If performing an event related paradigm, control the timing of your stimulus/event to happen just before the edit on acquisition of a MEGA-PRESS sequence. Taking elctrophysiological responses into account, 150 – 300 ms before data acquisition is likely a good estimate
- If a longer block design/Task is envisaged, then to maximise the GABA signal, it might be useful to just acquire EDIT ON sub-spectra across the block, and use the EDIT OFF sub-spectrum from the rest period for subtraction (while also acquiring some edit ON spectra during rest as well to provide a baseline). Note that, while this maximises the chance of detection, it will likely slightly over estimate the size of D[GABA]
- At the very least, know when the stimuli occurr relative to which sub spectrum (On or OFF) in the MEGA-PRESS acquisition, and ensure there are at least as many EDIT ON acquisitions as EDIT OFF, or even more EDIT ON to maximise chance of detection (again being aware of the slight over estimation of D[GABA] this may produce).
- Sliding window averaging should model the expected effects of increases (or decreases) in [GABA] occurring in mixed fashion across edit On and edit OFF acquisitions.
In conclusion, these simulations show that the timing of any expected GABA increases in relation to the edit ON and edit OFF portion of a MEGA-PRESS experiment can impact the amplitude of the resulting GABA peak in the EDITED subtraction spectrum. As a result, controlling, or being aware of the timing of stimulus onset in relation to the specific sub-spectra, or at least being aware of the timing, is likely to be crucial in event related fMRS paradigms using MEGA-PRESS for GABA.
References
Apšvalka, D., Gadie, A., Clemence, M., & Mullins, P. G. (2015). Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. NeuroImage, 118, 292–300. https://doi.org/10.1016/j.neuroimage.2015.06.015
Bednařík, P., Tkáč, I., Giove, F., DiNuzzo, M., Deelchand, D. K., Emir, U. E., Eberly, L. E., & Mangia, S. (2015). Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. Journal of Cerebral Blood Flow Metabolism, 35(4), 601–610. https://doi.org/10.1038/jcbfm.2014.233
Bednařík, P., Tkáč, I., Giove, F., Eberly, L. E., Deelchand, D. K., Barreto, F. R., & Mangia, S. (2017). Neurochemical responses to chromatic and achromatic stimuli in the human visual cortex. Journal of Cerebral Blood Flow Metabolism, 271678X17695291. https://doi.org/10.1177/0271678X17695291
Chen, C., Sigurdsson, H. P., Pépés, S. E., Auer, D. P., Morris, P. G., Morgan, P. S., Gowland, P. A., & Jackson, S. R. (2017). Activation induced changes in GABA: functional MRS at 7 T with MEGA-sLASER. NeuroImage, 156, 207–213. https://doi.org/10.1016/j.neuroimage.2017.05.044
Cleve, M., Gussew, A., & Reichenbach, J. R. (2014). In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. NeuroImage, 105(C), 1–9. https://doi.org/10.1016/j.neuroimage.2014.10.042
Evans, C. J., Puts, N. A. J., Robson, S. E., Boy, F., McGonigle, D. J., Sumner, P., Singh, K. D., & Edden, R. A. (2010). Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. Journal of Magnetic Resonance Imaging, 31(1), 204–209. https://doi.org/10.1002/jmri.21996
Ip, I. B., Berrington, A., Hess, A. T., Parker, A. J., Emir, U. E., & Bridge, H. (2017). Combined fMRI-MRS acquires simultaneous glutamate and BOLD-fMRI signals in the human brain. NeuroImage, 155, 1–19. https://doi.org/10.1016/j.neuroimage.2017.04.030
Koolschijn, R. S., Shpektor, A., Clarke, W. T., Ip, I. B., Dupret, D., Emir, U. E., & Barron, H. C. (2021). Memory recall involves a transient break in excitatory-inhibitory balance. ELife, 10, e70071. https://doi.org/10.7554/eLife.70071
Kurcyus, K., Annac, E., Hanning, N. M., Harris, A. D., Oeltzschner, G., Edden, R., & Riedl, V. (2018). Opposite Dynamics of GABA and Glutamate Levels in the Occipital Cortex during Visual Processing. The Journal of Neuroscience, 38(46), 9967–9976. https://doi.org/10.1523/JNEUROSCI.1214-18.2018
Lally, N., Mullins, P. G., Roberts, M. V., Price, D., Gruber, T., & Haenschel, C. (2014). Glutamatergic correlates of gamma-band oscillatory activity during cognition: A concurrent ER-MRS and EEG study. NeuroImage, 85 Pt 2, 823–833. https://doi.org/10.1016/j.neuroimage.2013.07.049
Mangia, S., Giove, F., Tkáč, I., Logothetis, N. K., Henry, P.-G., Olman, C. A., Maraviglia, B., Di Salle, F., & Ugurbil, K. (2008). Metabolic and hemodynamic events after changes in neuronal activity: Current hypotheses, theoretical predictions and in vivo NMR experimental findings. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 29(3), 441–463. https://doi.org/10.1038/jcbfm.2008.134
Matlab (Version 2022b). (2022). [MacOS 13.3.1]. The Mathworks Inc. https://www.mathworks.com
Mescher, M., Merkle, H., Kirsch, J., Garwood, M., & Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR IN BIOMEDICINE, 11, 7.
Mullins, P. G. (2018). Towards a theory of functional magnetic resonance spectroscopy (fMRS): A meta-analysis and discussion of using MRS to measure changes in neurotransmitters in real time. Scandinavian Journal of Psychology, 59(1), 91–103. https://doi.org/10.1111/sjop.12411
Mullins, P. G., McGonigle, D. J., O’Gorman, R. L., Puts, N. A. J., Vidyasagar, R., Evans, C. J., Cardiff Symposium on MRS of GABA, & Edden, R. A. E. (2014). Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage, 86, 43–52. https://doi.org/10.1016/j.neuroimage.2012.12.004
Mullins, P. G., Rowland, L. M., Jung, R. E., & Sibbitt, W. L. (2005). A novel technique to study the brain’s response to pain: Proton magnetic resonance spectroscopy. NeuroImage, 26(2), 642–646. https://doi.org/10.1016/j.neuroimage.2005.02.001
O’Gorman, R. L., Edden, R. A., Michels, L., Murdoch, J. B., & Martin, E. (2011). Precision and repeatability of in vivo GABA and glutamate quantification. Intl. Soc. Mag. Reson. Med., 3434.
O’Gorman, R. L., Michels, L., Edden, R. A., Murdoch, J. B., & Martin, E. (2011). In vivo detection of GABA and glutamate with MEGA-PRESS: Reproducibility and gender effects. Journal of Magnetic Resonance Imaging, 33(5), 1262–1267. https://doi.org/10.1002/jmri.22520
Pasanta, D., He, J. L., Ford, T., Oeltzschner, G., Lythgoe, D. J., & Puts, N. A. (2022). Functional MRS Studies of GABA and Glutamate/Glx – A Systematic Review and Meta-analysis. Neuroscience & Biobehavioral Reviews, 104940. https://doi.org/10.1016/j.neubiorev.2022.104940
Puts, N. A. J., & Edden, R. A. E. (2012). In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog NMR Spect, 60(C), 29–41. https://doi.org/10.1016/j.pnmrs.2011.06.001
Rogan, M., Friend, A. T., Rossetti, G. M., Edden, R., Mikkelsen, M., Oliver, S. J., Macdonald, J. H., & Mullins, P. G. (2022). Hypoxia alters posterior cingulate cortex metabolism during a memory task: A 1H fMRS study. NeuroImage,260, 119397. https://doi.org/10.1016/j.neuroimage.2022.119397
Simpson, R., Devenyi, G. A., Jezzard, P., Hennessy, T. J., & Near, J. (2015). Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magnetic Resonance in Medicine, 77(1), 23–33. https://doi.org/10.1002/mrm.26091
Stanley, J. A., Burgess, A., Khatib, D., Ramaseshan, K., Arshad, M., Wu, H., & Diwadkar, V. A. (2017). Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo 1H functional magnetic resonance spectroscopy. NeuroImage, 153, 189–197. https://doi.org/10.1016/j.neuroimage.2017.03.051
Taylor, R., Schaefer, B., Densmore, M., Neufeld, R. W. J., Rajakumar, N., Williamson, P. C., & Theberge, J. (2015). Increased glutamate levels observed upon functional activation in the anterior cingulate cortex using the Stroop Task and functional spectroscopy. Neuroreport, 26(3), 107–112. https://doi.org/10.1097/WNR.0000000000000309
Leave a comment